In the intricate tapestry of the pharmaceutical industry, where precision, innovation, and compliance intersect, the effective management of data emerges as a linchpin for success. The sector’s relentless pursuit of breakthrough therapies, stringent regulatory demands, and complex supply chains necessitate a robust framework for handling vast volumes of data. However, the industry grapples with a common challenge: data silos. These silos fragment critical information across various departments and systems, hindering collaboration, decision-making, and operational efficiency.
To navigate this complex landscape, pharmaceutical companies are turning to modern solutions like Boomi iPaaS (Integration Platform as a Service) to break down these data barriers and revolutionize their data management strategies. By leveraging Boomi’s advanced integration capabilities, pharmaceutical organizations can seamlessly integrate disparate data sources, streamline workflows, and unlock the full potential of their data to drive innovation and improve patient care. This blog post delves into the transformative impact of Boomi iPaaS on data management within the pharmaceutical sector, showcasing how it empowers companies to transcend data silos and excel in an era driven by data.
Maximizing Potential: The Critical Role of Data Management in the Pharma Industry
Before we dive in, let’s understand the importance of data management in the pharmaceutical industry. In an industry where research, development, manufacturing, and regulatory compliance are tightly intertwined, data serves as the lifeblood that fuels innovation and drives decision-making. Every stage of the pharmaceutical lifecycle, from drug discovery to commercialization and post-market surveillance, relies heavily on accurate, accessible, and well-managed data.
Effective data management ensures that researchers have access to the latest scientific insights, manufacturers can optimize production processes, regulatory bodies can monitor drug safety, and healthcare providers can deliver personalized patient care. Data also plays a pivotal role in every aspect of operations in the pharmaceutical industry, from research and development (R&D) to sales and marketing. With the sheer volume and complexity of data generated and consumed by pharmaceutical companies, the industry increasingly recognizes the need for centralized data management systems. These systems serve as the backbone for connecting all departments, facilitating seamless information sharing and automation across the organization.
Pharmaceutical companies that have embraced data management strategies are witnessing tangible benefits, including enhanced capacity to meet the needs of customers and stakeholders. These companies optimize processes from R&D to sales through automation and synchronized data sharing, improving efficiency and responsiveness.
Moreover, adopting pragmatic data management approaches is revolutionizing traditional practices within the pharmaceutical industry, particularly in research and drug development. By streamlining workflows and facilitating collaboration, these approaches drive innovation and result in significant cost savings.
Data management in the pharmaceutical sector encompasses various practice areas, each with its unique impact on operational efficiency:
- Big Data: Using massive datasets enables pharmaceutical businesses to detect patterns, insights, and trends that can improve decision-making and drive innovation in areas such as customized medicine and drug development.
- Data Analytics: Advanced analytics techniques enable pharmaceutical companies to extract valuable insights from data, from predicting patient outcomes to optimizing supply chain logistics.
- Data Warehouse: Centralized data warehouses provide a uniform repository for storing and analyzing large amounts of organized and unstructured data, allowing for more informed decision-making throughout the business.
- Data Quality: Ensuring data accuracy, completeness, and consistency is crucial for maintaining regulatory compliance and making reliable business decisions in the pharmaceutical industry.
- Master Data Management (MDM): MDM solutions help pharmaceutical companies maintain a single, authoritative source of truth for critical data entities such as products, customers, and regulatory information.
- Data Governance: Establishing robust frameworks ensures that data assets are managed, secured, and utilized effectively while mitigating data privacy risks and regulatory compliance risks.
Addressing Data Silos: Enhancing Data Integration in the Pharmaceutical Sector
Data silos present a pervasive challenge within the pharmaceutical industry, where various departments and functions often operate in isolation, leading to fragmented data landscapes. Research and development teams, manufacturing facilities, regulatory affairs departments, and commercial operations typically utilize separate systems and databases, resulting in disconnected data silos. These silos impede the flow of critical information across the organization, hindering collaboration, decision-making, and innovation. Moreover, data silos complicate efforts to maintain regulatory compliance as organizations struggle to reconcile and synchronize data across disparate systems. To overcome these challenges, pharmaceutical companies must prioritize data integration initiatives that break down silos and facilitate seamless information exchange across departments. By implementing robust data integration solutions and fostering a culture of collaboration, organizations can unlock the full potential of their data assets, driving operational excellence and accelerating scientific breakthroughs.
Revolutionizing Data Integration: Boomi iPaaS in the Pharma Industry
As pharmaceutical companies grapple with fragmented data landscapes and disparate systems, Boomi iPaaS offers a comprehensive solution to break down data silos and facilitate seamless information exchange. With its unified platform, Boomi enables organizations to connect diverse data sources, applications, and systems, regardless of location or format. Boomi’s extensive library of connectors supports integration with legacy systems, cloud-based applications, and emerging technologies, empowering pharmaceutical companies to leverage their data assets effectively.
Furthermore, Boomi’s advanced data transformation capabilities ensure that data is harmonized and standardized, enhancing accuracy and reliability across the organization. Boomi iPaaS enables pharmaceutical companies to drive innovation, improve operational efficiency, and deliver better patient outcomes by streamlining data integration processes and promoting cross-functional collaboration.
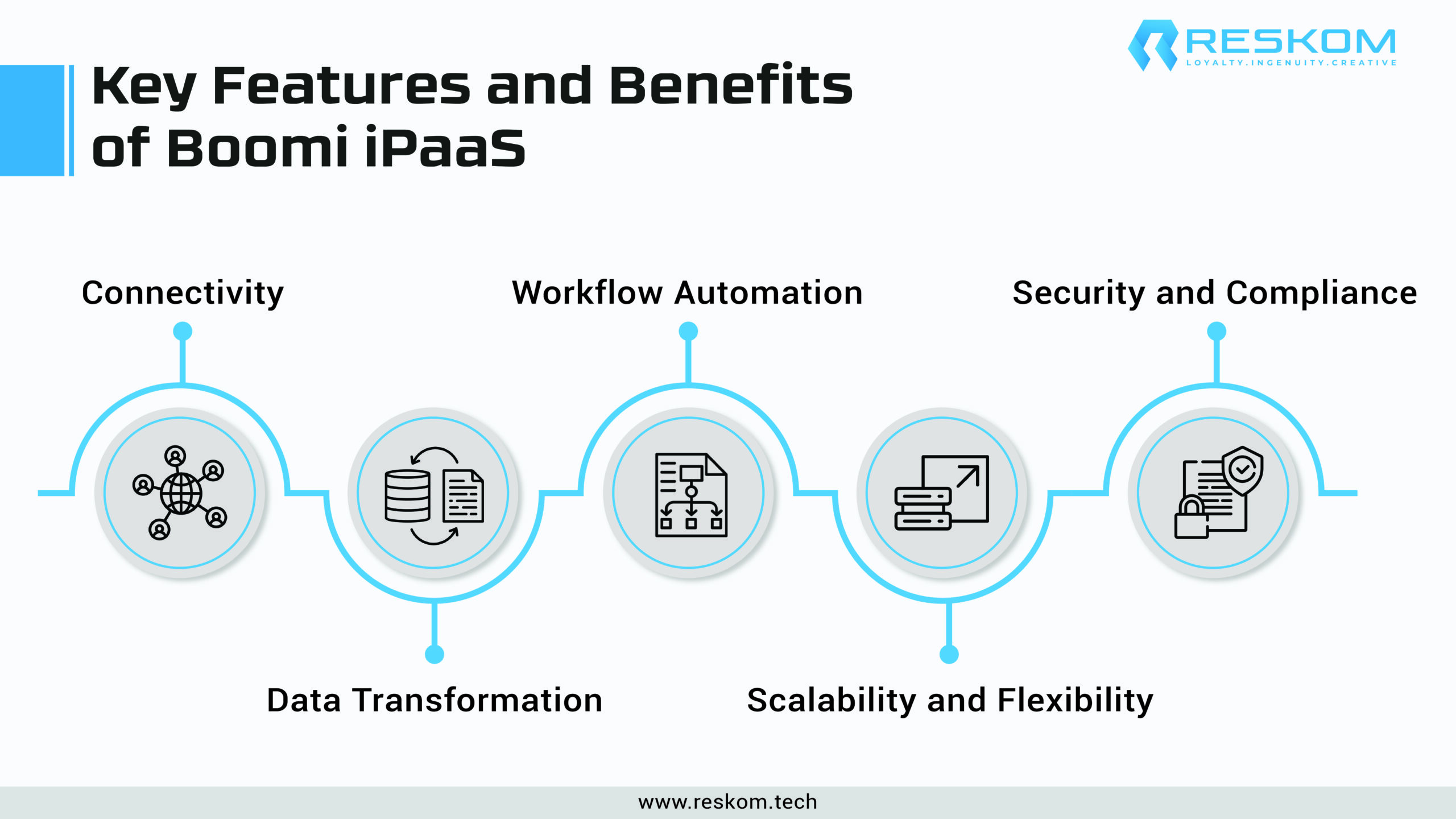
Key Features and Benefits of Boomi iPaaS: Empowering Data Integration in Pharma
Boomi’s iPaaS offers a suite of features tailored to address the unique data integration needs of the pharmaceutical industry, enabling organizations to streamline processes, enhance collaboration, and drive innovation. Here are some key features and benefits:
Comprehensive Connectivity: Boomi iPaaS provides a vast library of pre-built connectors, allowing pharmaceutical companies to seamlessly integrate data from disparate sources, including on-premises systems, cloud applications, and external partners. This comprehensive connectivity ensures that organizations can leverage their existing infrastructure while enabling new technologies and applications to be adopted.
Advanced Data Transformation: Boomi’s advanced data transformation capabilities enable organizations to harmonize and standardize data formats across diverse systems and applications. By facilitating data mapping, cleansing, and enrichment, Boomi iPaaS ensures data consistency and accuracy, empowering companies to gain insightful information and make well-informed decisions.
Workflow Automation: Boomi iPaaS empowers pharmaceutical companies to automate complex data processes, reducing manual effort and improving operational efficiency. With Boomi’s drag-and-drop interface and customizable workflows, organizations can automate data integration, validation, and synchronization tasks, enabling seamless collaboration and accelerating time-to-insight.
Scalability and Flexibility: Boomi’s cloud-native architecture offers scalability and flexibility, allowing organizations to adapt seamlessly to changing business needs and data volumes. Whether scaling up to accommodate growing data volumes or integrating new applications and systems, Boomi iPaaS provides the agility and resilience needed to support evolving business requirements.
Security and Compliance: Boomi prioritizes data security and regulatory compliance, providing robust encryption, access controls, and audit trails to safeguard sensitive information. With Boomi’s comprehensive security features, including role-based access controls and data masking, pharmaceutical companies can ensure compliance with industry regulations while protecting sensitive patient data.
In summary, breaking down data silos is essential for driving innovation, ensuring compliance, and maintaining a competitive edge in the pharmaceutical industry. Boomi iPaaS offers pharmaceutical companies a powerful solution for transforming data management practices, enabling seamless integration and collaboration across the organization. By embracing Boomi iPaaS, pharmaceutical companies can uncover the full potential of their data assets and drive meaningful advancements in drug discovery, development, and commercialization, ultimately improving patient care and driving business success.


