Regulatory compliance is essential to the pharmaceutical industry for patient safety and for the integrity of the industry as a whole; it is more than simply meeting guidelines. The pharma industry is confronted with the formidable challenge of keeping up with the ever-increasing complexity of compliance requirements while maintaining operational efficiency and innovation.
According to recent industry data, the pharmaceutical sector has paid over $50 billion in compliance-related penalties since 2000. This substantial investment underscores the critical importance of compliance in the pharmaceutical sector and the need for streamlined processes to manage regulatory requirements effectively.
Within the scope of this blog, we investigate how Boomi’s Integration Platform as a Service (iPaaS) helps pharmaceutical businesses overcome the difficulties associated with compliance, thereby enabling them to navigate the regulatory landscape with simplicity and confidence.
Compliance Complexities and Challenges in Pharma
Pharmaceutical companies operate in a highly regulated environment, with strict guidelines and standards enforced by regulatory bodies such as the Central Drug Standards Control Organization (CDSCO) in India, the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in Europe, and the World Health Organization (WHO). These regulations govern different areas of drug development, manufacturing, distribution, and marketing to assure product safety, efficacy, and quality.
One of the primary compliance challenges in the pharmaceutical industry is the need to maintain data integrity and traceability throughout the product lifecycle. This includes:
- Managing electronic records and signatures
- Maintaining documentation for audits and inspections
- Ensuring compliance with good manufacturing practices (GMP) and good laboratory practices (GLP).
- Adverse event reporting
- Intellectual property protection
- Regulatory compliance in research and development
Moreover, pharmaceutical companies must navigate complex supply chains involving multiple stakeholders, including suppliers, manufacturers, distributors, and healthcare providers. Each entity may have its own set of compliance requirements, making it challenging to achieve seamless integration and data exchange across the supply chain network.
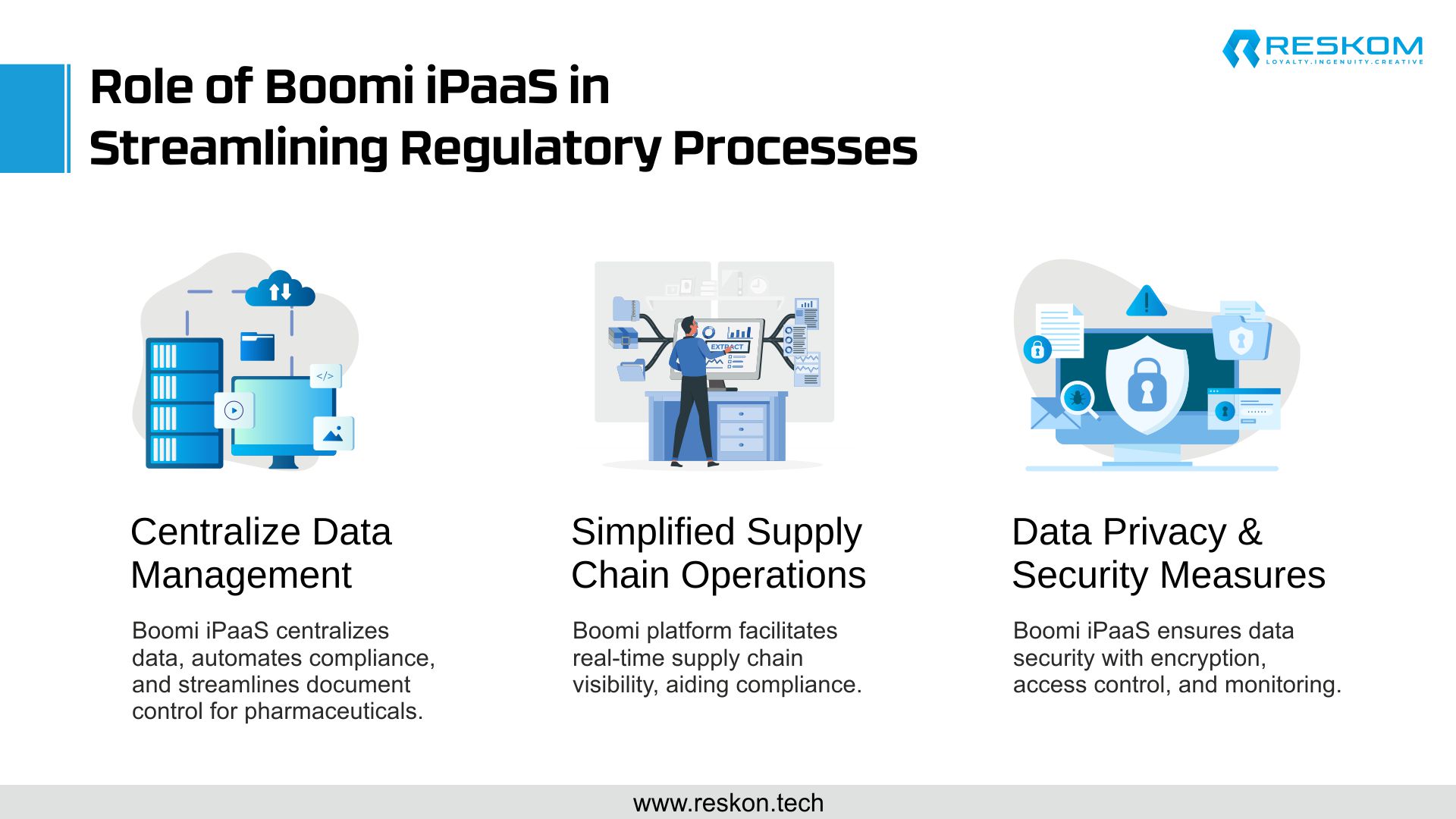
The Role of Boomi iPaaS in Streamlining Regulatory Processes
Boomi iPaaS offers a comprehensive integration solution designed to address the unique compliance needs of the pharmaceutical industry. By leveraging Boomi’s platform, companies can achieve seamless connectivity and data integration across disparate systems, applications, and partners while ensuring compliance with regulatory standards.
- Centralize Data Management: One of the key advantages of Boomi iPaaS is its ability to centralize data management and automate critical compliance processes. With Boomi, pharmaceutical companies can streamline document control, electronic signature management, and audit trail generation, reducing the risk of compliance violations and costly penalties.
- Simplified Supply Chain Operations: Boomi’s platform also enables real-time visibility into supply chain operations, allowing companies to track the movement of raw materials, components, and finished products across the entire supply chain ecosystem. This enhanced visibility helps identify potential compliance issues proactively and take corrective actions before they escalate into major compliance breaches.
- Data Privacy & Security Measures: Furthermore, Boomi iPaaS provides robust security features, including data encryption, role-based access control, and activity monitoring, to safeguard sensitive information and ensure compliance with data privacy regulations such as the Health Insurance Portability and Accountability Act (HIPAA) and the General Data Protection Regulation (GDPR).
Case Study: Compliance with DSCSA (USA) requirements with Boomi AtomSphere
Healthcare consumers often take daily medication without considering the intricate processes behind ensuring its safety and journey through the supply chain. For Pharma companies, adhering to the Drug Supply Chain Security Act (DSCSA) presents a significant challenge, aiming to combat counterfeit drugs and facilitate recalls since its inception in 2013. Compliance involves tracking prescription drugs across the supply chain, requiring comprehensive data management and transparency.
Several challenges arise in meeting DSCSA requirements, particularly for startups and small to midsize pharma businesses (SMBs), including:
- A wide partner ecosystem necessitates secure data exchange.
- Requirement for unit-level track and trace, increasing complexity.
- Implementation of secure data standards like EPCIS for product integrity.
Noncompliance not only risks financial penalties but also brand damage and litigation. Yet, compliance efforts offer opportunities for enhanced efficiency and insights, such as:
- Increased visibility across the supply chain.
- Improved inventory management and cost efficiency.
- Streamlined trading partner relationships.
Boomi AtomSphere provides a robust platform for integration, vital for building track and trace capabilities compliant with DSCSA. Jade Global, a Boomi Elite Partner, specializes in modernizing and scaling track and trace systems for startups and SMBs, leveraging Boomi’s low-code platform to accelerate implementation by 25 to 30%.
Integration with ERP and third-party track and trace systems, facilitated by Boomi, ensures secure data exchange and compliance reporting. Such systems enable continuous supply chain optimization, offering visibility into functions like planning, procurement, and logistics. Moreover, they facilitate EDI capabilities for seamless partner transactions and onboarding.
A cloud-native integration platform like Boomi AtomSphere ensures adaptability to evolving industry landscapes, including emerging technologies like blockchain for track and trace. With Boomi and Jade Global’s joint solutions, pharmaceutical companies can navigate compliance requirements while preparing for future innovations in supply chain management.
Closing words
In conclusion, pharmaceutical businesses must pay close attention to regulatory compliance, risk management, and process efficiency. Boomi iPaaS helps pharmaceutical firms overcome regulatory issues by integrating, managing data, and automating processes.
Pharmaceutical firms can focus on innovation and providing safe, high-quality goods to patients worldwide by using Boomi’s platform to improve agility, efficiency, and compliance. Boomi iPaaS helps pharmaceutical firms turn regulatory compliance into a strategic advantage, boosting corporate growth and competitive differentiation in the dynamic market.
Have any thoughts on your mind? Share with us! Contact our Boomi platform strategist to discuss your challenges and find a customized solution.