Research and development in the pharmaceutical sector is undergoing transformative shifts due to data-driven decision-making and interconnected systems. Amidst the quest for novel treatments and therapies, the integration of disparate data sources and the seamless flow of information has become imperative.
Boomi Integration Platform as a Service (iPaaS) emerged as a pivotal enabler, revolutionizing how pharmaceutical companies conduct research, collaborate, and innovate. This blog post explores the multifaceted role of Boomi iPaaS in driving innovation within pharma research, ultimately transforming the landscape of clinical trials.
The Evolution of Clinical Trials
Clinical trials are the linchpin of pharmaceutical research, serving as the conduit through which promising drug candidates are rigorously evaluated for safety, efficacy, and regulatory compliance. Historically, these trials have been marked by cumbersome processes, siloed data systems, and fragmented communication channels, leading to inefficiencies, delays, and escalating costs. Moreover, the exponential growth of data volumes and stringent regulatory requirements have compounded these challenges, necessitating a paradigm shift in trial management strategies.
The Role of Integration in Pharma Research
Integration lies at the heart of modernizing clinical trials, enabling seamless connectivity and data exchange across disparate platforms, applications, and stakeholders. By bridging the gap between electronic health records (EHRs), laboratory information management systems (LIMS), patient recruitment platforms, and regulatory databases, integration streamlines trial workflows, enhances data accuracy, and ensures real-time visibility into study progress.
However, traditional integration approaches, characterized by custom code development and point-to-point connections, are inherently complex, costly, and time-consuming, impeding agility and innovation. Here is a summary of how integration contributes to Pharma research:
- Enhances collaboration between pharmaceutical researchers and regulatory bodies.
- Optimizes data flow and accessibility across pharmaceutical research platforms.
- Fosters interoperability among diverse data sources in pharmaceutical research.
- Facilitates real-time monitoring and analysis of pharmaceutical research data.
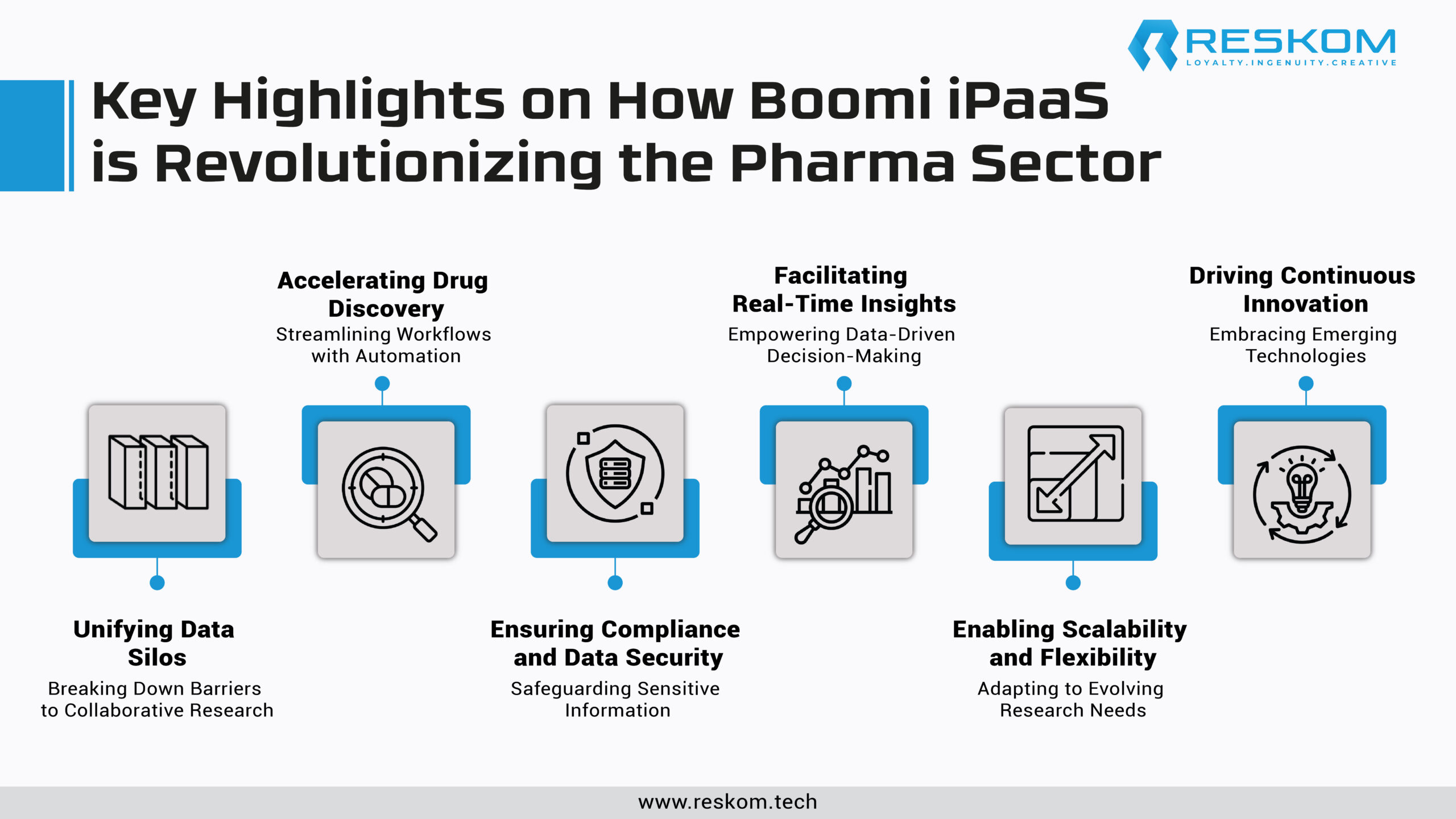
Unifying Data Silos: Breaking Down Barriers for Collaborative Research
In the realm of pharmaceutical research, valuable insights often lie buried within disparate data silos, ranging from clinical trials and genomics to patient records and real-world evidence. Boomi iPaaS is a unifying force, seamlessly integrating data from diverse sources, including legacy systems, cloud applications, and IoT devices. By breaking down silos and establishing data harmony, Boomi empowers researchers to access a comprehensive view of information, fostering collaboration and cross-functional insights crucial for driving innovation.
Accelerating Drug Discovery: Streamlining Workflows with Automation
The journey from drug discovery to market approval entails intricate workflows involving numerous stakeholders and processes. Boomi iPaaS streamlines these workflows through intelligent automation, orchestrating data flows, and triggering actions based on predefined rules.
By automating repetitive tasks such as data validation, transformation, and synchronization, Boomi expedites the drug discovery process, enabling researchers to focus their efforts on critical analysis and experimentation. This acceleration not only enhances productivity but also expedites time-to-market for life-saving medications.
Ensuring Compliance and Data Security: Safeguarding Sensitive Information
In the highly regulated landscape of pharma research, compliance with stringent data privacy and security standards is paramount. Boomi iPaaS offers robust capabilities for ensuring compliance at every stage of the data lifecycle. Through built-in governance features, encryption mechanisms, and role-based access controls, Boomi safeguards sensitive information, ensuring adherence to regulations such as HIPAA and GDPR.
By providing a secure and auditable framework for data integration, Boomi instills confidence among stakeholders and mitigates risks associated with data breaches and regulatory non-compliance.
Facilitating Real-Time Insights: Empowering Data-Driven Decision-Making
In the dynamic landscape of pharmaceutical research, real-time access to insights is indispensable for informed decision-making. Boomi iPaaS enables the seamless flow of data in real time, empowering researchers with up-to-date information for analysis and decision support.
Whether monitoring patient outcomes in clinical trials or tracking adverse events, Boomi facilitates the timely dissemination of actionable insights, driving agility and responsiveness in research initiatives. Pharmaceutical companies can pivot quickly in response to emerging trends and opportunities by harnessing the power of real-time data integration and analytics.
Enabling Scalability and Flexibility: Adapting to Evolving Research Needs
The scalability and flexibility of research infrastructure are critical for accommodating evolving scientific paradigms and business requirements. Boomi iPaaS offers a cloud-native architecture designed to scale seamlessly with growing data volumes and user demands. Whether expanding research operations globally or integrating new data sources, Boomi provides the agility to adapt quickly without compromising performance or reliability. By decoupling integration processes from the underlying infrastructure, Boomi empowers pharmaceutical companies to embrace innovation without the constraints of traditional IT environments.
Driving Continuous Innovation: Embracing Emerging Technologies
Innovation in pharmaceutical research extends beyond conventional boundaries, embracing emerging technologies such as artificial intelligence, machine learning, and blockchain. Boomi iPaaS serves as a catalyst for integrating these technologies into research workflows, enabling seamless interoperability and data exchange.
Whether leveraging AI algorithms for predictive analytics or deploying blockchain for secure data sharing, Boomi provides the foundation for driving continuous drug discovery and development innovation. By embracing emerging technologies within a unified integration framework, pharmaceutical companies can unlock new possibilities for therapeutic advancements and patient care.
Final Thoughts
As the pharma industry navigates the complexities of modern research, the role of Boomi iPaaS emerges as indispensable in driving innovation and advancing scientific discovery. By unifying data silos, streamlining workflows, ensuring compliance, facilitating real-time insights, enabling scalability, and embracing emerging technologies, Boomi empowers pharmaceutical companies to accelerate the pace of innovation and bring life-saving treatments to market more efficiently.
As research paradigms evolve, Boomi remains at the forefront, shaping the future of pharmaceutical research through seamless integration and collaboration. Have any thoughts? Contact Boomi experts at RESKOM for a more detailed discussion on how we leverage Boomi iPaaS for your Pharma venture.


